Suppose the reaction 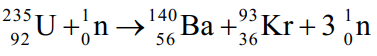 produces 1.664 *1010 kJ/mol of energy.
produces 1.664 *1010 kJ/mol of energy.
Calculate the change in mass in grams that occurs when one mole of U-235 reacts with one mole of neutrons.E = mc2, where c = 2.998 *108 m/s; 1 kg = 6.0221415 *1026 amu; 1 J = 1kg · m2/s2.
A) 0.185 g
B) 0.555 g
C) 0.898 g
D) 5.41 g
E) 9.22 F*10-17 g
Correct Answer:
Verified
Q73: Which statement is NOT correct? During hydrogen
Q74: What forces must be overcome for any
Q76: Which of the following statements regarding uranium
Q79: Which statement is NOT correct? During primordial
Q80: The quantity of material required to ensure
Q81: If boron-10 is bombarded with a neutron,
Q82: If a nitrogen-14 nuclide captures an
Q83: Radionuclide A has a half-life that is
Q109: The difference between the uranium used in
Q126: Nuclear fission produces energy because _
A)neutrons are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents