In the figure shown, what does the dotted line represent? 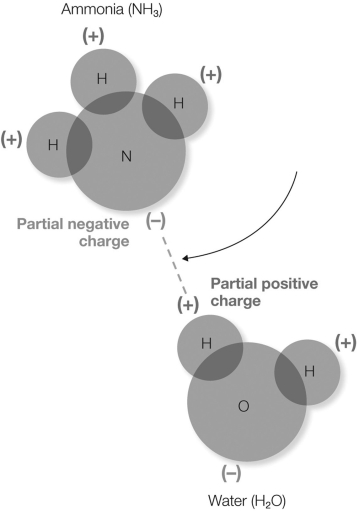
A) transfer of the electron from the hydrogen atom to the nitrogen atom
B) sharing of electrons between the ammonia and water molecules
C) a Van der Waals interaction
D) an electrostatic interaction between the partially- positive hydrogen and the partially- negative nitrogen
E) an interaction between a hydrophilic and a hydrophobic molecule
Correct Answer:
Verified
Q1: Isotopes are atoms of the same element
Q2: A feature of many of the isotopes
Q4: A compound which stabilizes pH by absorbing
Q5: In a polar covalent bond,
A) electrons are
Q6: Which of the following is incorrectly matched?
A)
Q7: Compared to a solution with a pH
Q8: An atom is best described as
A) the
Q9: The pictured molecules both contain six carbon
Q10: Which of the following shows an ionic
Q11: What information can you determine about the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents