How much energy is required to remove a neutron (mn = 1.008 665 u) from 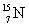 that has an atomic mass of 15.000 108 u to make
that has an atomic mass of 15.000 108 u to make 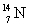 that has an atomic mass of 14.003 074 u? Note: The energy equivalent of the atomic mass unit is 931.5 MeV.
that has an atomic mass of 14.003 074 u? Note: The energy equivalent of the atomic mass unit is 931.5 MeV.
A) 1.163 MeV
B) 6.423 MeV
C) 10.83 MeV
D) 928.7 MeV
E) 939.6 MeV
Correct Answer:
Verified
Q7: Which one of the following statements concerning
Q8: How many neutrons are there in the
Q8: Which process is used in the operation
Q9: Complete the following sentence: In a
Q10: Assuming the radius of a hydrogen atom
Q13: The binding energy of an isotope of
Q15: Radiation or(and) particles emerge(s) from a
Q16: Which one of the following types
Q27: An isotope of krypton has a half-life
Q32: The half-life of a particular isotope of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents