The same activity is measured for two different isotope samples.One sample contains 0.0450 kg of 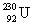 (atomic mass = 230.033 937 u, t1/2 = 20.8 days) .The second sample contains an unknown amount of
(atomic mass = 230.033 937 u, t1/2 = 20.8 days) .The second sample contains an unknown amount of 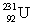 (atomic mass = 231.036 264 u, t1/2 = 4.3 days) .What is the mass of the second sample?
(atomic mass = 231.036 264 u, t1/2 = 4.3 days) .What is the mass of the second sample?
A) 0.0093 kg
B) 0.23 kg
C) 0.110 kg
D) 0.037 kg
E) 0.0450 kg
Correct Answer:
Verified
Q21: Which process is involved in determining the
Q23: Determine the activity of 6.0 × 1012
Q24: Which one of the following statements
Q26: If the uranium nucleus is at rest
Q27: Determine the amount of energy released in
Q28: The ratio of the abundance of carbon-14
Q29: Tritium is an isotope of hydrogen
Q31: Determine the amount of energy released in
Q35: In a certain
Q37: A sample contains 1000 nuclei of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents