Complete the following statement: An h subshell refers to orbital quantum number
A) 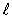 = 1.
= 1.
B) 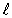 = 2.
= 2.
C) 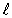 = 3.
= 3.
D) 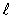 = 4.
= 4.
E) 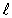 = 5.
= 5.
Correct Answer:
Verified
Q22: How many electrons could be accommodated in
Q24: According to the quantum mechanical picture of
Q26: Which quantum number applies to most of
Q29: An electron in a hydrogen atom is
Q31: Which one of the following factors best
Q33: What energy (in eV) is required to
Q35: Which one of the following subshells is
Q36: To which model of atomic structure does
Q37: Determine the maximum number of electron states
Q39: According to the quantum mechanical picture of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents