Complete the following statement: The first law of thermodynamics states that
A) heat is a form of energy.
B) entropy is a function of state.
C) the entropy of the universe is increasing.
D) the change in the internal energy of a system is given by 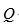 - W.
- W.
E) no engine can be more efficient than a Carnot engine operating between the same two temperatures.
Correct Answer:
Verified
Q14: 5.00 kg of liquid water is heated
Q15: Complete the following statement: Walls that separate
Q16: An isobaric process is represented on a
Q17: Rick spends four hours researching on the
Q18: A match ignites within in an oxygen-filled
Q20: 5.00 kg of liquid water is heated
Q21: A quantity of carbon monoxide gas is
Q22: Two moles of an ideal gas have
Q23: Beneath the moveable piston in the drawing,2.25
Q24: An ideal monatomic gas undergoes an adiabatic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents