A thermally isolated sample of an ideal gas at a fixed temperature is confined to one half of a container by an impermeable membrane.The other half of the container is evacuated.The membrane is then pierced and the gas is allowed to expand freely and to double its volume as shown.Which one of the following statements is true concerning this situation? 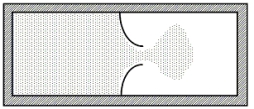
A) The process is reversible.
B) This is an isothermal process.
C) The entropy of the gas decreases.
D) The internal energy of the gas must decrease.
E) The temperature of the gas decreases to one-half of its original value.
Correct Answer:
Verified
Q5: Neon is a monatomic gas with a
Q7: A system containing an ideal gas at
Q11: An ideal gas absorbs 750 J of
Q12: Which one of the following pressure-volume graphs
Q13: Enclosed beneath the moveable piston in the
Q14: 5.00 kg of liquid water is heated
Q16: An isobaric process is represented on a
Q18: A match ignites within in an oxygen-filled
Q24: A quantity of carbon monoxide gas is
Q27: An ideal monatomic gas originally in state
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents