A paddle wheel frictionally adds thermal energy to an ideal monatomic gas in a sealed, insulated container.The paddle wheel is driven by a cord connected to a falling object as shown in the drawing.In this experiment, a 5.0-kg object falls through a total distance of 3.0 m and the temperature of the gas is found to increase by 6 C°.Assume that all of the mechanical energy lost by the falling object goes into the gas.How many moles of gas must be present in this container? 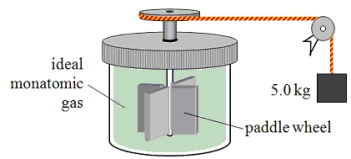
A) 2.0
B) 3.0
C) 4.0
D) 5.0
E) 6.0
Correct Answer:
Verified
Q24: An ideal monatomic gas undergoes an adiabatic
Q28: A paddle wheel frictionally adds thermal energy
Q29: In an isothermal process,1.59 moles of an
Q30: One mole of a monatomic gas at
Q33: An ideal gas is taken from state
Q33: A fixed amount of ideal gas is
Q34: Two moles of a confined ideal monatomic
Q35: An ideal monatomic gas originally in state
Q36: Two moles of an ideal gas have
Q40: During one stage of a reversible process,the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents