Heat is added to a 1.0-kg solid sample of a material at -200 °C. The figure shows the temperature of the material as a function of the heat added. 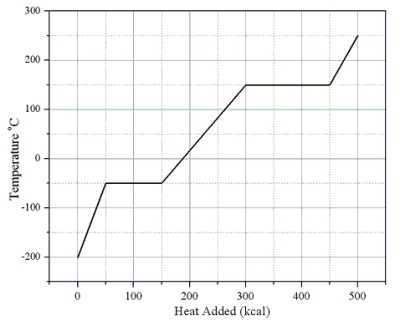
-What is the specific heat capacity of this substance in its solid state?
A) 0.33 cal/(g · C°)
B) 0.75 cal/(g · C°)
C) 1.00 cal/(g · C°)
D) 1.33 cal/(g · C°)
E) 3.00 cal/(g · C°)
Correct Answer:
Verified
Q56: In an insulated container, 0.50 kg of
Q57: Given the following information, determine the relative
Q58: Ryan places 0.150 kg of boiling water
Q59: Which would cause a more serious burn:
Q60: A 0.030-kg ice cube at 0 °C
Q61: After working a 0.55-kg iron horseshoe with
Q62: Heat is added to a 1.0-kg solid
Q63: Heat is added to a 1.0-kg solid
Q64: Heat is added to a 1.0-kg solid
Q66: Heat is added to a 1.0-kg solid
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents