Multiple Choice
Are all the carbons in this structure in the same plane? 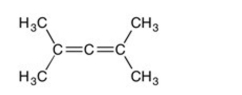
A) Yes
B) No
Correct Answer:
Verified
Related Questions
Q26: How many sp2 hybridized atoms are in
Q27: Which carbons in the following compound are
Q28: How many carbon-carbon sigma bonds are in
Q29: Each lone pair on the CH3OH occupies
Q30: Of the hydrogen halides, the strongest bond
Q32: How many sp2 carbons are present in
Q33: CH3CN contains _ sigma bonds and _
Q34: What orbitals overlap to form the H-C
Q35: The nitrogen atom in (CH3CH2)3N _ is
Q36: How many carbon-carbon sigma bonds are in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents