Using the symbols δ+ and δ- , show the direction of the polarity in the O-H bond. 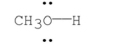
Correct Answer:
Verified
Q37: Which of the following angles is closest
Q38: What orbitals overlap to form the C-H
Q39: How many sp-hybridized atoms are in the
Q40: The lone-pair electrons of the methyl anion
Q41: Which of the following compounds does
Q43: Which bond in the following compound is
Q44: What two factors determine the size of
Q45: What is the H-C-H bond angle in
Q47: Convert the following Kekulé structure into a
Q69: The carbon-carbon double bond in ethene is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents