A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle, as shown in the pV diagram in the figure. If the process is carried out in a clockwise sense around the enclosed area, as shown on the figure, then the change of internal energy over the full cycle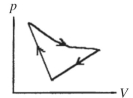
A) is zero.
B) is positive.
C) is negative.
D) cannot be determined from the information given.
Correct Answer:
Verified
Q300: A battery charges a parallel-plate capacitor fully
Q301: The work done on an ideal gas
Q302: A solid aluminum cube rests on a
Q303: During an isochoric process, the internal (thermal)
Q304: The potential difference between the plates of
Q306: An ideal gas is compressed isobarically to
Q307: A conductor is placed in a steady
Q308: A negatively-charged plastic rod is brought close
Q309: The second law of thermodynamics leads us
Q310: In a given reversible process, the temperature
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents