The figure shows a diagram for of ideal nitrogen gas in a sealed container. The temperature of state 1 is , the atomic mass of the nitrogen atom is , and - K. What are (a) pressure and (b) temperature ?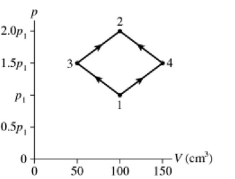
A) (a) , (b)
B) (a) 81 atm, (b)
C) (a) , (b)
D) (a) , (b)
Correct Answer:
Verified
Q6: A certain gas is compressed adiabatically.
Q7: The figure shows a
Q8: The figure shows a
Q9: The process shown on the
Q10: Which of the following is a
Q12: When water at
Q13: The temperature of an ideal gas
Q14: An ideal gas undergoes an isothermal expansion.
Q15: The process shown on the
Q16: The figure shows a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents