FIGURE 20-2 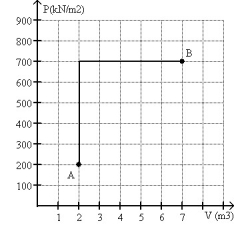
-A monatomic ideal gas reversibly follows the process shown in Fig. 20-2 starting from state A at a temperature 300 K and ending at state B.
(a) What is the final temperature of the gas?
(b) What is the change in internal energy of the gas in going from state A to state B?
(c) What is the change in entropy of the gas in going from state A to state B?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q19: Sketch the schematic diagram of energy transfers
Q20: No device is possible whose sole effect
Q21: The coefficient of performance (COP) of a
Q23: Which of the following is an example
Q25: From the energy that is input into
Q27: Natural processes tend to move toward a
Q28: A engine manufacturer makes the claim that
Q29: A Carnot cycle consists of
A)two adiabats and
Q46: Which of the following is a statement
Q54: One of the most efficient engines built
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents