FIGURE 20-3 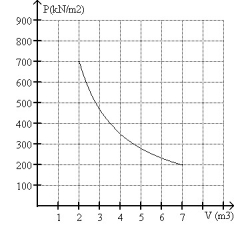
-What is the change in entropy of 10.0 moles of monatomic ideal gas with a molar specific heat 3R/2 that reversibly undergoes the isothermal expansion shown in Fig. 20-3?
A) 104 J/K
B) 63.1 J/K
C) 45.2 J/K
D) 90.8 J/K
E) The answer cannot be determined without knowing the temperature of the gas.
Correct Answer:
Verified
Q2: A certain engine extracts 1300 J of
Q37: A Carnot cycle engine operates between a
Q56: A perfect Carnot engine operates between 350
Q58: A heat engine operates between 800 K
Q62: The temperature inside a Carnot refrigerator placed
Q63: An 800-g block of ice at 0.00°C
Q64: A 3.00-kg block of silicon at 60.0°C
Q65: 1.0 kg of steam at 100°C condenses
Q66: 24.0 moles of a diatomic ideal gas
Q93: A heat engine operating at maximum efficiency
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents