The image shows bonding for diamond on the left) and graphite on the right) . Both minerals are made of carbon, so why do they have very different properties why is diamond so hard while graphite is so soft) ? 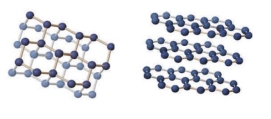
A) Covalent bonds in both minerals are very strong, but intermolecular bonds between sheets in graphite are very weak.
B) Graphite's mixture of covalent and intermolecular bonds is stronger than the solely covalent bonds within diamond.
C) Diamond has a different type of covalent bonds between the atoms of carbon than does graphite.
D) Minerals with only one type of bond will always be harder than minerals that have two different types of bonding.
Correct Answer:
Verified
Q96: Which of the following pairs of minerals
Q97: Which layer in the earth has a
Q98: Minerals that do not contain silicon are
Q99: The two elements most abundant in the
Q100: Which of the following minerals are common
Q102: Which side of this molecule has a
Q103: In which of the following types of
Q104: When halite dissolves in water the:
A) chlorine
Q105: In which of the following types of
Q106: Using the Periodic Table, what can you
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents