Would this crossed Aldol reaction work well? Why or why not?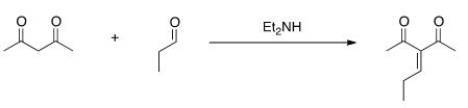
A) Yes, the diketone is significantly more acidic, so this enolate can be formed selectively.
B) Yes, the aldehyde is significantly more acidic, so this enolate can be formed selectively.
C) No, the aldehyde is significantly more acidic, so this enolate cannot be formed selectively.
D) No, the diketone is significantly more acidic, so this enolate cannot be formed selectively.
Correct Answer:
Verified
Q21: What is the name given to the
Q24: In a Michael reaction,what is the name
Q28: Of the carbonyl compounds; (1) benzaldehyde,
Q29: Which of the following compounds can undergo
Q32: Under basic conditions, the Aldol reaction
Q32: What is the general name of the
Q34: What are the two starting materials for
Q37: What is an intramolecular Claisen reaction called?
A)Michael
Q38: What is the product of the following
Q40: In a Michael reaction,where does the nucleophile
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents