Starting with cyclohexanone, how could you prepare the diketone below?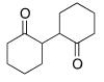
A) Treat cyclohexanone with a base under thermodynamic conditions.
B) Hydrogenate cyclohexanone with Raney nickel.
C) Convert cyclohexanone into the a-bromoketone and then react this with the enolate of cyclohexanone.
D) Convert cyclohexanone into an enamine with diethylamine and then react this with more cyclohexanone.
Correct Answer:
Verified
Q3: What is the starting material in the
Q3: Will acetophenone be completely deprotonated by lithium
Q4: What is the starting material for the
Q5: Why is the enolate of acetone less
Q9: What is the missing reagent for the
Q10: Which of the following is an enol
Q11: What are the three steps in the
Q11: Which is the kinetic enolate of 2-methylcyclohexanone?
Q12: Which of the following four compounds is
Q15: Why is it difficult to stop the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents