Which of the following statements is (are) true about the IR spectrum of the compound drawn below?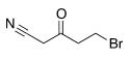
A) It shows absorptions at 3000-3150 cm-1 and 1720 cm-1.
B) It shows absorptions at 3000-2850 cm-1 and 2150 cm-1.
C) It shows absorptions at 2250 cm-1 and 1650 cm-1.
D) It shows absorptions at 2250 cm-1 and 1720 cm-1.
E) Both statements B (It shows absorptions at 3000-2850 cm-1 and 2250 cm-1) and D (It shows absorptions at 2250 cm-1 and 1720 cm-1) are true.
Correct Answer:
Verified
Q7: Which of the following statements is
Q8: Stronger bonds will be found where in
Q9: Which of the following statement(s) is
Q10: In electron impact mass spectrometry (EIMS),what is
Q14: Why is the infrared absorption for the
Q15: What type(s)of molecular motion is (are)observed using
Q15: Which of the following statements is
Q17: You observe a compound that exhibits
Q19: What type of feature will be observed
Q19: Compared to a C-H bond,a C-D bond
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents