What is the hybridization for each of the indicated atoms in the following compound?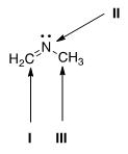
A) I = sp2; II = sp2; III = sp2.
B) I = sp2; II = sp3; III = sp3.
C) I = sp; II = sp2; III = sp3.
D) I = sp2; II = sp2; III = sp3.
Correct Answer:
Verified
Q43: What is the condensed formula of the
Q44: When forming molecular orbitals from atomic
Q45: Which atomic orbitals overlap to form the
Q49: Which of the following is the appropriate
Q50: Convert the following skeletal structure to a
Q54: Which atomic orbitals overlap to form the
Q60: What is the hybridization of the carbon
Q70: Which of the following statements about electronegativity
Q71: Which atomic orbitals overlap to form the
Q74: Which molecule has the greatest difference in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents