Which of the following processes results in the conversion of a neutron to a proton within a nucleus?
A) alpha particle emission
B) beta ( 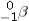 ) particle emission
) particle emission
C) positron emission
D) neutron emission
E) proton emission
Correct Answer:
Verified
Q1: Which of the following forms of radiation
Q3: Rank the following in order of increasing
Q5: Which process decreases the atomic number of
Q6: Which of the following statements regarding nuclear
Q7: Gamma rays are
A)high-energy electromagnetic radiation.
B)H nuclei.
C)electrons.
D)He nuclei.
E)positrons.
Q10: Alpha particles are equivalent to
A)He atoms.
B)H atoms.
C)electrons.
D)positrons
E)He
Q12: Which of the following statements regarding nuclear
Q13: Which process decreases the atomic number of
Q16: An alpha particle has a mass number
Q17: Which of the following forms of radiation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents