Identify the particle emitted by the nucleus that undergoes the transformation shown in the figure. 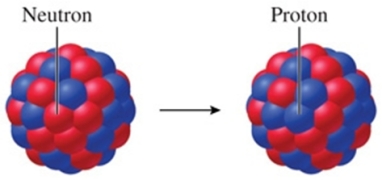
A) an alpha particle
B) a beta particle (electron)
C) a positron
D) a gamma ray
E) a neutron
Correct Answer:
Verified
Q33: Bombardment of boron-10 with a neutron produces
Q34: Select the most stable isotope from the
Q35: Protactinium-234 decays by beta emission to form
Q36: Potassium-40 decays by beta emission to form
Q37: Predict the type of radiation emitted when
Q39: Thorium-234 decays by beta emission to form
Q41: What type of radioactive decay would be
Q44: What type of radioactive decay would be
Q65: Carbon-11 radioactively decays by positron emission with
Q67: Carbon-14 has a half-life of 5730 yr.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents