What product is formed when 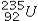 undergoes 2 alpha emissions and 2 beta emissions in a total of four steps?
undergoes 2 alpha emissions and 2 beta emissions in a total of four steps?
A) actinium-227
B) thorium-227
C) thorium-231
D) polonium-231
E) radon-227
Correct Answer:
Verified
Q57: The half-life of iodine-123 is 13.3 hours.
Q60: The half-life of iodine-131 is 8.1 days.
Q61: What product is formed when 
Q62: What is product of the first step
Q64: What product is formed when 
Q66: Which of the following isotopes would be
Q68: Carbon-11 radioactively decays by positron emission with
Q74: Which of the following statements is incorrect?
A)Pacemakers
Q84: The amount of time that it takes
Q100: Alpha particles are the least harmful type
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents