Consider a voltaic cell that corresponds to the following reaction: Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) If this reaction takes place in the electrochemical cell shown in the figure, which of the following statements is incorrect? 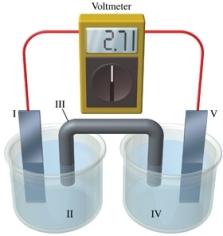
A) I is the zinc electrode, which is the anode.
B) II is the Cu2+ solution.
C) III is the salt bridge.
D) IV contains the substance that is being reduced.
E) V is the copper electrode, which is the cathode.
Correct Answer:
Verified
Q44: Consider the reaction: Zn(s) + H2SO4(aq)
Q45: Consider the reaction: Sn2+(aq) + 2Fe3+(aq)
Q46: Given the following reaction in a
Q47: Consider the reaction: Cu(s) + 4HNO3(aq)
Q50: Consider a voltaic cell that corresponds
Q51: Consider a voltaic cell that corresponds
Q52: In a voltaic cell, the electron flow
Q52: Consider the reaction: Cu(s) + 4HNO3(aq)
Q53: Consider the reaction: Cu(s) + 4HNO3(aq)
Q54: Consider the reaction: Sn2+(aq) + 2Fe3+(aq)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents