Consider a voltaic cell that corresponds to the following reaction: Mg(s) + Sn2+(aq) Mg2+(aq) + Sn(s) If this reaction takes place in the electrochemical cell shown in the figure, which of the following statements is incorrect? 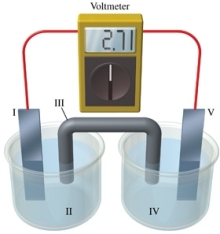
A) I is the magnesium electrode, which is the anode.
B) II is the Mg2+ solution.
C) III is the salt bridge.
D) IV contains the substance that is being oxidized.
E) V is the tin electrode, which is the cathode.
Correct Answer:
Verified
Q54: Consider the reaction: Sn2+(aq) + 2Fe3+(aq)
Q55: Consider the reaction: Zn(s) + H2SO4(aq)
Q56: In a fuel cell that uses
Q57: Consider the reaction: Zn(s) + H2SO4(aq)
Q58: Consider a voltaic cell that corresponds
Q60: Consider the reaction: Sn2+(aq) + 2Fe3+(aq)
Q61: A voltaic cell is prepared in
Q62: A mercury button battery that is
Q63: The reaction that occurs in an
Q64: The reaction that occurs in an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents