The figure shows the electrolysis of molten AlCl3.What is the balanced equation for the reaction? 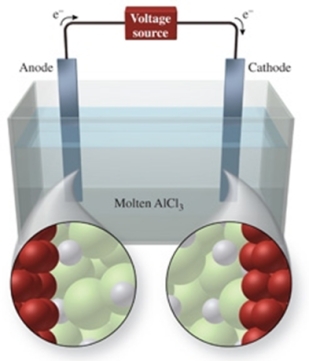
A) AlCl3(l) Al3+(l) + 3Cl-(l)
B) AlCl3(l) Al(l) + Cl3(g)
C) 2AlCl3(l) 2Al(l) + 3Cl2(g)
D) AlCl3(s) Al3+(aq) + 3Cl-(aq)
E) 2AlCl3(s) 2Al(s) + 3Cl2(s)
Correct Answer:
Verified
Q88: In any electrolytic cell, the cathode is
Q91: Given the following information about the
Q92: The figure shows the electrolysis of
Q93: The figure shows the electrolysis of
Q94: Given the following information about the
Q96: Consider the reaction: Al(s) + H2O(l)
Q97: Consider the half-reaction ClO-(aq)
Q98: Given the following information about the
Q99: Consider the reaction: H3AsO3(aq) + BiO3-(aq)
Q100: Consider the half-reaction NH4+(aq)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents