Consider the reaction CO(g) + H2O(g) CO2(g) + H2(g) , represented by the following diagram: 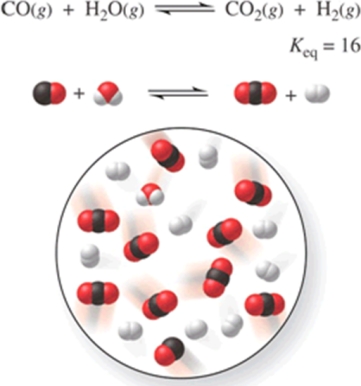 What is the composition of this system when the reaction reaches equilibrium?
What is the composition of this system when the reaction reaches equilibrium?
A) 3 CO, 3 H2O, 7 CO2, 7 H2
B) 1 CO, 1 H2O, 4 CO2, 4 H2
C) 2 CO, 2 H2O, 8 CO2, 8 H2
D) 8 CO, 8 H2O, 2 CO2, 2 H2
E) 4 CO, 6 H2O, 4 CO2, 6 H2
Correct Answer:
Verified
Q4: Factors that influence reaction rates include all
Q6: A catalyst speeds up a chemical reaction
Q16: Identify an intermediate in the following
Q18: Identify the intermediate(s) in the following
Q21: When a chemical system has reached equilibrium
A)the
Q24: Which equilibrium constant represents a reaction that
Q25: The graph shows the change in concentrations
Q37: Given that evaporation is an endothermic process,
Q51: Which of the following statements is correct
Q84: The amount of energy that reactant molecules
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents