The two balloons shown in the figure each have the same volume, temperature, and pressure.Which of the following statements regarding the two balloons is correct? 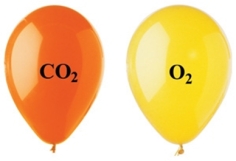
A) The CO2 balloon has fewer molecules than the O2 balloon.
B) The density of the O2 balloon is greater than the density of the CO2 balloon.
C) The mass of the O2 balloon is greater than the mass of the CO2 balloon.
D) The CO2 balloon is more buoyant than the O2 balloon.
E) None of these statements is correct.
Correct Answer:
Verified
Q44: Calculate the number of moles and the
Q49: Calculate the number of moles in
Q50: Calculate the number of moles in 55
Q51: Calculate the number of moles in 75
Q52: In January a balloon is taken
Q52: Calculate the number of moles and the
Q55: Calculate the number of moles and the
Q55: Calculate the number of moles and the
Q57: A sample of gas initially occupies 2.50
Q59: A balloon is filled with helium at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents