In the figure shown, is a chemical reaction occurring? 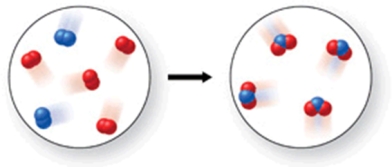
A) No, because there are the same number of atoms in both images.
B) Yes, because the atoms have rearranged, and therefore the formulas of the products and reactants are different.
C) No, because there are oxygen atoms and nitrogen atoms in both images.
D) No, because both the reactants and products are colorless gases.
E) Yes, because the reactants are gases, but the product is a solid.
Correct Answer:
Verified
Q14: Which of the following changes represents a
Q15: Fireworks which give off bright flashes
Q16: Consider the following chemical equations.Select the equations
Q17: The figure shows a reaction between xenon
Q18: Consider the following chemical equations.Select the equations
Q21: When the equation shown is balanced
Q22: Balance the following skeletal equation: Ba(NO3)2(aq)
Q23: After the following equation is properly
Q25: The gases carbon dioxide and hydrogen can
Q29: A reaction which has two elements as
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents