In the figure shown, is a chemical reaction occurring? 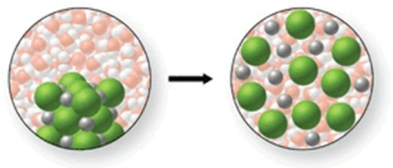
A) Yes, because the Na+ and Cl- ions are being removed from their ionic lattice as they are dissolved.
B) Yes, because the water molecules are reacting with the Na+ and Cl- ions to form a gas.
C) Yes, because a precipitate will be formed when the water and NaCl are mixed.
D) No, because there is no change occurring.
E) No, because the Na+ and Cl- ions are simply being surrounded by the water molecules as the salt dissolves.
Correct Answer:
Verified
Q7: Gaseous nitrogen monoxide reacts with oxygen gas
Q8: Identify which image in the figure represents
Q9: Consider the following chemical equations.Select the equations
Q10: The figure shows a reaction between hydrogen
Q11: Sulfur dioxide gas reacts with oxygen gas
Q13: Write a complete, balanced equation for
Q14: The figure shows the chemical reaction between
Q15: Fireworks which give off bright flashes
Q16: Consider the following chemical equations.Select the equations
Q17: The figure shows a reaction between xenon
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents