Write a balanced equation to represent the reaction shown in the figure. 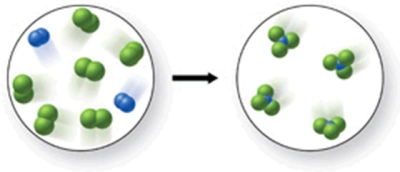
A) 2N2 + 6Cl2 3NCl4
B) N2 + Cl2 2NCl3
C) 2N2 + 6Cl2 4NCl3
D) N2 + 6Cl2 4NCl3
E) 2N2 + 5Cl2 3NCl3
Correct Answer:
Verified
Q21: When the equation shown is balanced
Q22: Balance the following skeletal equation: Ba(NO3)2(aq)
Q23: After the following equation is properly
Q25: The gases carbon dioxide and hydrogen can
Q27: After the following equation is properly
Q28: Balance the following skeletal equation: CH4(g)
Q29: A reaction which has two elements as
Q29: Write a balanced equation to represent
Q30: Balance the following skeletal equation: C3H8(g)
Q33: A solution of silver nitrate is mixed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents