The class of the reaction shown in the figure is: 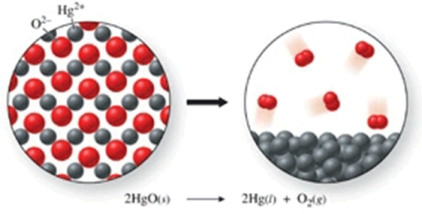
A) double-displacement reaction.
B) combination reaction.
C) combustion reaction.
D) decomposition reaction.
E) single-displacement reaction.
Correct Answer:
Verified
Q34: A reaction which has two compounds as
Q36: Balance the following skeletal equation: Li(s)
Q37: Balance the following skeletal equation: C2H5OH(l)
Q38: Balance the following skeletal equation: Pb(NO3)2(aq)
Q39: Balance the following skeletal equation: HCl(g)
Q42: The class of the reaction shown in
Q44: When aqueous solutions of H2SO4 and NaOH
Q45: Write and balance the equation for
Q47: Crystals of red mercury(II)oxide, when heated, form
Q53: Sodium metal reacts with water in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents