Rank the substances in the figure from least atoms per mole to most atoms per mole. 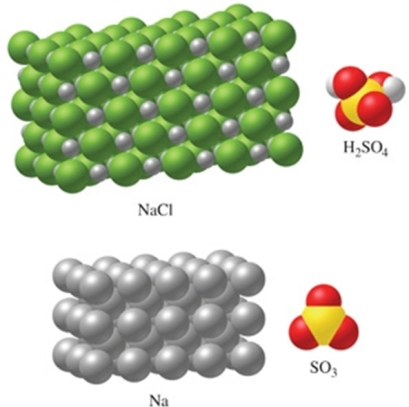
A) SO3 < H2SO4 < NaCl < Na
B) Na < NaCl < SO3 < H2SO4
C) Na < NaCl < H2SO4 < SO3
D) NaCl < Na < SO3 < H2SO4
E) H2SO4 < SO3 < NaCl < Na
Correct Answer:
Verified
Q21: If 4.05 x 1023 molecules of a
Q22: How many hydrogen atoms are there in
Q23: Rank the following in order of increasing
Q24: Calculate the molar mass of CuSO4.5H2O.
A)159.62 g/mol
B)249.70
Q26: Which of the following statements regarding atoms,
Q27: How many oxygen atoms are present in
Q28: Rank the following in order of increasing
Q29: Calculate the molar mass of PH3.
A)40.11 g/mol
B)31.98
Q30: Calculate the molar mass of HCl.
A)36.46 g/mol
B)13.02
Q31: Rank the substances in the figure from
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents