Which of the substances in the figure have the same empirical and molecular formulas? 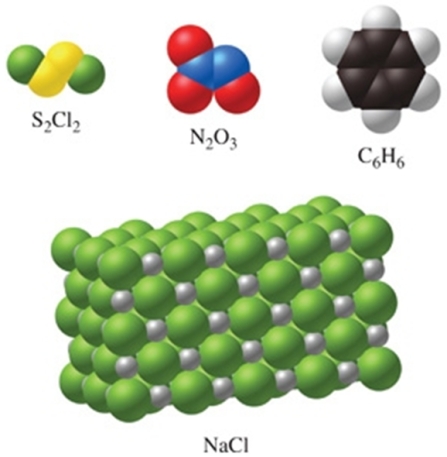
A) S2Cl2 and C6H6
B) none of the substances
C) all of the substances
D) N2O3 and NaCl
E) N2O3 only
Correct Answer:
Verified
Q50: A chemical reaction requires 3.50 moles of
Q56: If the molar mass of a substance
Q57: If you have 10.0 g of sodium
Q58: Calculate the number of moles of NaOH
Q60: Calculate the moles of sucrose, C12H22O11, in
Q62: Methyl butyrate is a compound that is
Q64: Which of the following statements regarding empirical
Q65: How many moles are in a sample
Q69: Which of the following statements regarding empirical
Q77: Which of the following statements regarding empirical
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents