How much water had to be added to 10.0 mL of the first solution to obtain the second solution? 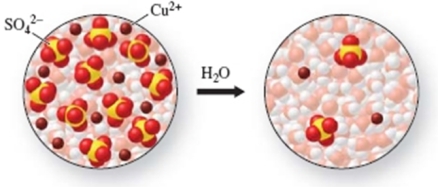
A) 10.0 mL
B) 20.0 mL
C) 40.0 mL
D) 50.0 mL
E) 500.0 mL
Correct Answer:
Verified
Q83: Calculate the moles and the mass of
Q84: In order to prepare a 0.0500 M
Q85: Calculate the moles and the mass of
Q86: Calculate the moles and the mass of
Q87: Acetic acid has a molar mass of
Q89: Calculate the molarity of a solution consisting
Q90: Calculate the molarity of a solution consisting
Q92: Calculate the molarity of a solution consisting
Q93: How much water must be added to
Q114: Calculate the volume of 14.0 M HNO3
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents