The figure shows a molecular-level diagram of the chemical reaction between hydrogen and nitrogen to form ammonia.What is wrong with this diagram? 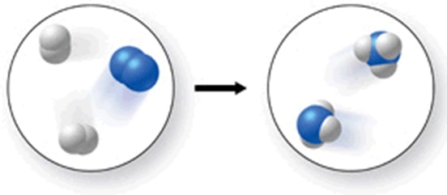
A) The products contain more nitrogen atoms than the reactants.
B) The products contain more hydrogen atoms than the reactants.
C) The number of reactant molecules should equal the number of product molecules.
D) The products should contain some unreacted hydrogen.
E) The product ammonia molecules should have only two hydrogen atoms attached to nitrogen.
Correct Answer:
Verified
Q1: The number of _ determines the identity
Q2: The subatomic particles that make up the
Q3: Which of the following statements regarding atomic
Q4: Which of the following were defined as
Q6: An atom contains
A)as many neutrons as electrons.
B)as
Q9: Which of the following statements regarding atoms
Q11: Rutherford's scattering experiment demonstrated
A)the existence of protons.
B)the
Q14: Which of the following is not part
Q17: Which particles are found in the atomic
Q19: Which of the following statements is incorrect?
A)The
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents