The isotope symbol for an ion that has 13 protons, 14 neutrons, and 10 electrons is:
A) 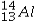
B) 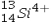
C) 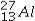
D) 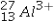
E) none of these
Correct Answer:
Verified
Q40: The correct isotope symbol for the isotope
Q42: Which of the following contains 18 neutrons?
A)(31P)
B)(34S2-
Q42: Which of the following statements about Mendeleev's
Q43: The isotope symbol for an ion that
Q44: Which of the following statements about the
Q46: A horizontal row of elements in the
Q47: The isotope symbol for an ion that
Q49: Which of the following does not apply
Q52: How many protons, neutrons, and electrons are
Q55: Which of the following statements does not
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents