Does the figure shown represent a chemical change or a physical change, and does it obey the law of conservation of mass? 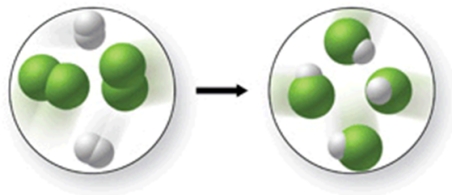
A) chemical change; law of conservation of mass is obeyed
B) chemical change; law of conservation of mass is not obeyed
C) physical change; law of conservation of mass is obeyed.
D) physical change; law of conservation of mass is not obeyed
Correct Answer:
Verified
Q84: To which class does the element calcium
Q86: Calculate the relative atomic mass of speedium
Q88: Calcium citrate is a compound found in
Q92: The correct symbol for the ion formed
Q94: Which of the following elements does not
Q95: Which of the following best describes the
Q96: Which of the following has the same
Q98: Which of the following does not have
Q99: Select the element that is an alkali
Q100: Which of the following elements does not
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents