What is the atomic number, mass number, and charge, respectively, of the atom or ion represented? 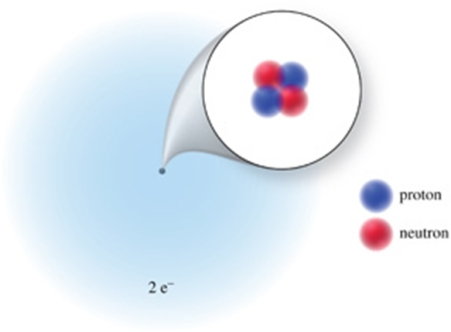
A) 2, 2, 2-
B) 2, 4, 2+
C) 2, 4, 0
D) 2, 2, 0
E) 2, 6, 4-
Correct Answer:
Verified
Q69: To the correct number of significant figures,
Q70: Which of the following statements regarding ion
Q73: Which set of elements below contains, respectively,
Q74: Elements in Group VIIIA (18)are called:
A)halogens.
B)noble gases.
C)alkali
Q80: Elements in Group VIIA (17)are called:
A)halogens.
B)chalcogens.
C)noble gases.
D)inert
Q85: To which class does the element chromium
Q86: The correct symbol for the ion formed
Q87: Select the element that is an alkaline
Q89: The ions of most main-group elements have
Q90: In which group of the periodic table
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents