A student wrote the following electron configuration for a ground state, neutral nitrogen atom: 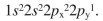 Explain why the configuration does not describe the lowest energy state of a ground-state nitrogen atom and provide the lowest-energy electron configuration for nitrogen.
Explain why the configuration does not describe the lowest energy state of a ground-state nitrogen atom and provide the lowest-energy electron configuration for nitrogen.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q29: Explain what is meant by the term
Q30: Define the term node as it applies
Q31: Which of the following pairs are not
Q32: What is the relationship between the principal
Q33: Which of the following statements is true
Q35: Which of these orbital interactions would be
Q36: How many molecular orbitals are generated from
Q37: How many values can ml have for
Q38: Write the lowest-energy electron configuration for a
Q39: Each of the chemical events shown represents
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents