For each molecule shown, indicate whether the molecule is polar by drawing a dipole arrow  pointing towards the negative end of the molecule.
pointing towards the negative end of the molecule.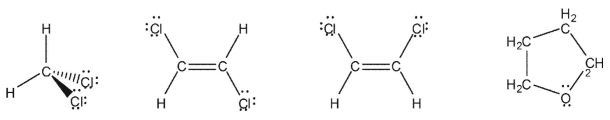
Correct Answer:
Verified
Q38: Write the lowest-energy electron configuration for a
Q39: Each of the chemical events shown represents
Q40: State the Heisenberg uncertainty principle.
Q41: Draw a Lewis structure for methyl anion,
Q42: Is forming a bond between an oxygen
Q44: The carbon-nitrogen bond in formamide, HCONH2, has
Q45: The Lewis structure of the anion shown
Q46: Using curved arrow formalism, show the homolytic
Q47: Use an orbital interaction diagram to provide
Q48: Draw Lewis structures for the following compounds.Show
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents