Beryllium hydride (BeH2) is a linear molecule with two perpendicular p-orbitals on the beryllium atom: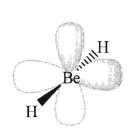
An s-orbital approaching BeH2 will only be able to interact with one of the two p-orbitals; explain why.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q44: The carbon-nitrogen bond in formamide, HCONH2, has
Q45: The Lewis structure of the anion shown
Q46: Using curved arrow formalism, show the homolytic
Q47: Use an orbital interaction diagram to provide
Q48: Draw Lewis structures for the following compounds.Show
Q50: In the orbital interaction diagram for H2
Q51: Which of the following resonance structures is
Q52: Draw a Lewis structure for methyl cation,
Q53: Using the Lewis structure of acetaldehyde shown,
Q54: Draw a Lewis structure for acetamide, CH3CONH2.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents