For compounds like CH3OCH2Cl, it has been determined that the gauche conformation shown here is lower in energy than the anti conformation, a phenomenon called the anomeric effect. One explanation for the anomeric effect is like the one provided for the lower energy of the staggered conformation of ethane relative to eclipsed ethane. This explanation invokes a stabilizing interaction between an empty orbital and a filled orbital in the gauche conformation of the molecule. The Newman projections shown here look down the central C-O bond: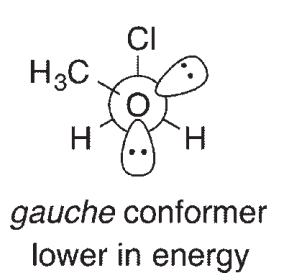
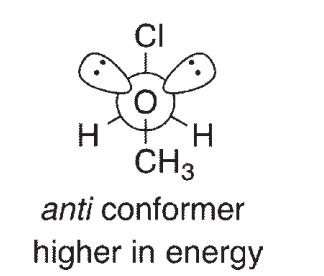
One of the orbitals involved in this theory contains one of the oxygen lone pairs.What is the other
orbital?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q36: Provide the hybridization for the indicated atoms
Q37: What orbitals are involved from carbon and
Q38: Which of the following molecules will have
Q39: How many 13C signals will be
Q40: What is the approximate hybridization of each
Q42: The hydrocarbon 2,2,4-trimethylpentane is a popular "antiknock"
Q43: The reaction shown here is one you
Q44: Draw a structure corresponding to the systematic
Q45: Label each atom indicated as primary
Q46: Write a systematic name for the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents