Assuming 98.85 % of the molecules are in the conformation with the group A equatorial, what is the value of ΔG° for this equilibrium at 25°C? R=1.987 x10-3 kcal/mol K 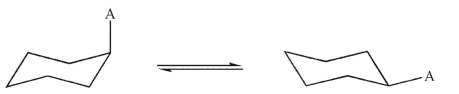
A) -2.64 kcal/mol
B) -2.72 kcal/mol
C) 0.228 kcal/mol
D) 2.64 kcal/mol
E) 2.72 kcal/mol
Correct Answer:
Verified
Q3: Using the heats of combustion per CH2
Q4: What is the highest-energy conformation of cyclohexane?
A)chair
B)full
Q5: Which of the following molecules is chiral?
A)
Q6: Which of the following words describes a
Q7: How many signals would appear in the
Q9: Which double Newman projection matches the view
Q10: Which of these cycloalkanes is planar in
Q11: What percentage of methylcyclohexane exists in the
Q12: For which of the following do you
Q13: Consider the two chair conformations of trans-1,3-dimethylcyclohexane.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents