Boiling point increases as shown in the following series of amines: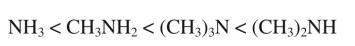
Why is the boiling point of (CH3) 3N lower than that of (CH3) 2NH?
A) (CH3) 2NH has a higher molar mass than (CH3) 3N .
B) (CH3) 2NH can form hydrogen bonds, but (CH3) 3 N cannot.
C) (CH3) 3 N can form hydrogen bonds, but (CH3) 2NH cannot.
D) (CH3) 2NH is polar, but (CH3) 3 N is not.
E) (CH3) 3N is polar, but (CH3) 2NH is not.
Correct Answer:
Verified
Q20: Which of these structures is ethylene glycol?
A)
Q21: Provide the IUPAC name for the molecule
Q22: Place the following molecules in order of
Q23: Provide the IUPAC name for this molecule,
Q24: Which of the following statements about organolithium
Q26: Provide a common name for the molecule
Q27: Which of the following compounds do you
Q28: Draw a correct structure for morpholine.
Q29: Provide the IUPAC name for the structure
Q30: Which of these types of compounds is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents