In the gas phase, the order of acidity of the alcohols shown here is reversed. Explain the difference in acidity trends between the gas phase and solution. 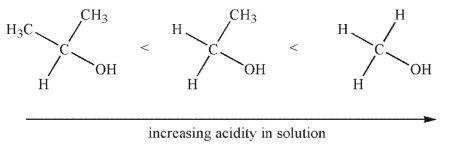
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q41: Why is ethanol a stronger acid than
Q42: Which of these compounds is more acidic,
Q43: Which has a higher boiling point, ethanol
Q44: Draw the mechanism showing the Brønsted acid/base
Q45: What is the common name of this
Q47: Predict the product of the following reaction
Q48: Distinguish between protic solvents and aprotic solvents
Q49: Draw a mechanism for the Brønsted acid/base
Q50: Draw the conjugate acid and the conjugate
Q51: In the following compounds, identify which proton
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents