For the reaction profile shown below, which statement(s) could be made? 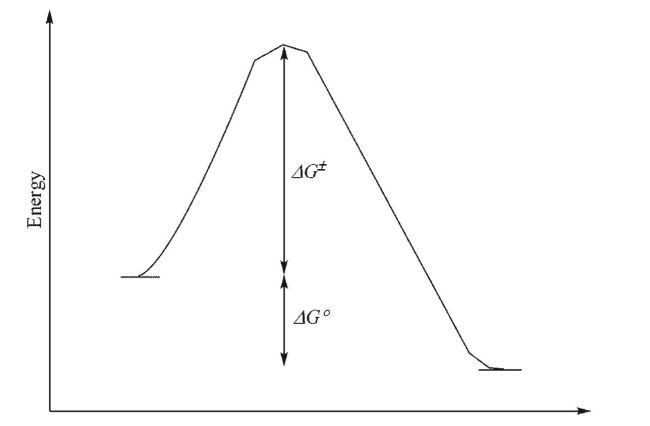
I.The transition state will look more like the starting materials than the products.
II.The reaction is endothermic.
III.The equilibrium constant for the forward reaction will be larger than 1.
IV.The activation barriers for the forward and reverse reactions are equal.
A) I only
B) I and II
C) I and III
D) I, II and III
E) I, III and IV
Correct Answer:
Verified
Q7: Identify the product of the following reaction.
Q8: Which of the following is the product
Q9: Which of the following would react most
Q10: Which of these choices is the best
Q11: Which of the following would not be
Q13: For the following reaction, what will be
Q14: Which of the following factors would not
Q15: Identify the HOMO and the LUMO
Q16: Which of the following electrophiles would undergo
Q17: Which of the following ideas best explains
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents