What will happen to the rate of the reaction below if the concentration of the bromide is tripled? 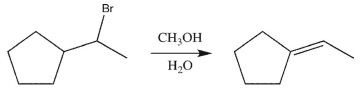
A) The rate will double.
B) The rate will be cut in half.
C) The rate will decrease by a factor of one-third.
D) The rate will increase by a factor of nine.
E) The rate will triple.
Correct Answer:
Verified
Q1: What will happen to the rate of
Q2: Which of the following carbocations is (are)
Q4: Which is the most likely product of
Q5: What is the major SN1 product of
Q6: Ehats the major E1 product of this
Q7: Which of these is the most stable
Q8: What will happen to the rate of
Q9: What is the major E1 product of
Q10: Which of the following statements is true?
A)The
Q11: Which of the following intermediates has the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents