Consider the energy diagram for the multistep reaction shown here.Which statement about this diagram is not true? 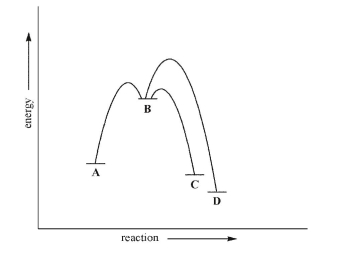
A) C is the kinetic product.
B) D is the thermodynamic product.
C) Given sufficient energy, C and D can equilibrate.
D) Formation of D is under thermodynamic control.
E) Formation of C is under thermodynamic control.
Correct Answer:
Verified
Q13: Which is the least likely product of
Q14: What is the major product of this
Q15: In which of these reactions do you
Q16: What will happen to the rate of
Q17: Which of these structures shows the preferred
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents