What would be the effect on the 1H NMR spectrum of adding a drop of D2O to a sample of the molecule shown here?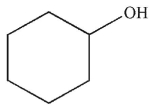
A) Adding the D2O will cause more hydrogen bonding and will change the chemical shift of the hydroxyl proton.
B) Adding the D2O will cause less hydrogen bonding and will change the chemical shift of the hydroxyl proton.
C) A new signal for D2O will appear in the 1H NMR spectrum.
D) A deuterium atom will exchange for the hydroxyl proton and the signal in the 1H NMR spectrum will disappear.
E) Adding D2O will have no effect on the 1H NMR spectrum.
Correct Answer:
Verified
Q31: A common fragmentation pattern of cyclohexene derivatives
Q32: Draw the products of McLafferty rearrangement of
Q33: Which hydrogen would have a signal farthest
Q34: Explain why there is always an M
Q35: Which hydrogen would have a signal farthest
Q37: How many "lines" do you expect in
Q38: Which of the following compounds corresponds to
Q39: The molecule shown here was analyzed by
Q40: Which structure matches the 1H
Q41: Are the two hydrogens indicated homotopic, enantiotopic,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents